Overview
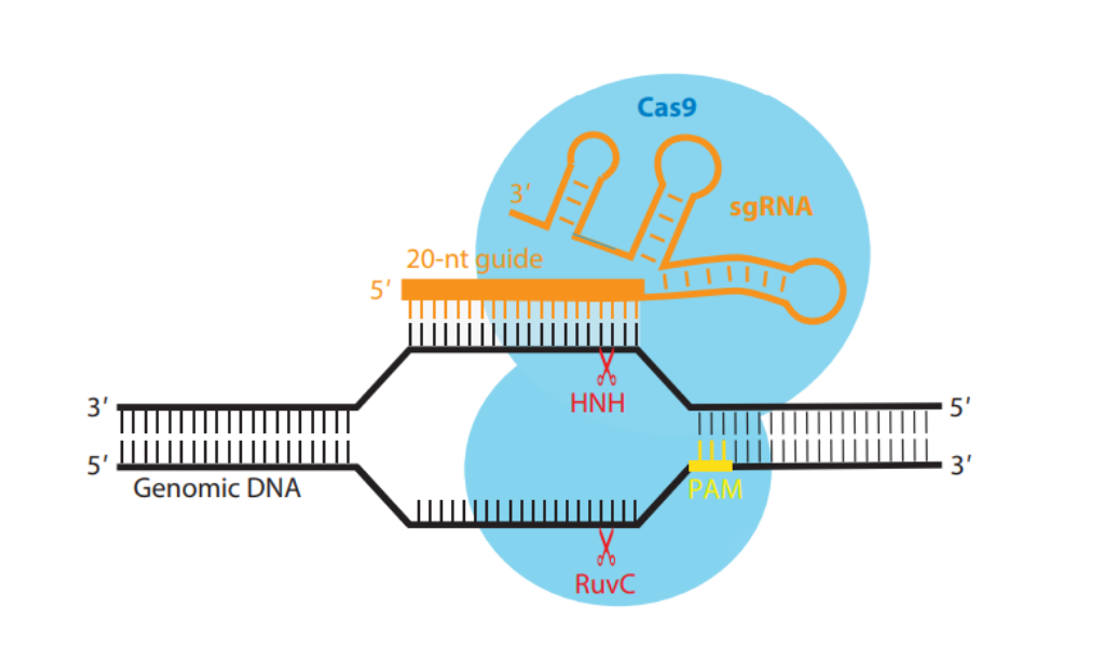
In November of this year, the Medicines and Health Products Regulatory Authority (MHRA) in the UK approved a CRISPR/Cas9 gene editing therapy for sickle cell disease, which caused quite a sensation at the time. It should be noted that this was the world’s first approved CRISPR therapy.
Although CRISPR technology has been highly sought after before, it has not been able to enter clinical practice due to various reasons, and the announcement of this news has attracted global attention.
Less than a month later, good news came again, and on December 8th, the US Food and Drug Administration (FDA) followed suit and approved the Casgevy treatment method.
Many researchers see this event as a “historic moment” and may become a turning point for CRISPR technology from 1.0 to 2.0.
With the emergence of different gene editing tools, “this will mean different diseases, and each patient can explore different methods.”
On November 16th, UK regulatory authorities approved a treatment method using gene editing technology, which inactivates specific genes to treat hereditary blood disease sickle cell disease. This caused a considerable sensation at the time, as it was the world’s first approved CRISPR/Cas9 gene editing therapy.

However, researchers are clearly not satisfied with this and hope to obtain a second authorization. This time, they are targeting a larger market – the United States. On December 8th, less than a month later, this gene editing therapy was approved by the FDA.

It can be said that this is a major event in the field of gene editing therapy. Many people see it as a turning point in the era of gene editing technology from 1.0 to 2.0.
At present, there are various therapies in the field of gene editing, but this may only be the beginning.
Keith Gottesdiener, CEO of Prime Medicine, said, “We tend to call these genome editing 1.0… they can do some amazing things, but their application scope is relatively limited.”
The era of genome editing 2.0 is on its way, and a new generation of CRISPR systems can overcome the shortcomings of previous technologies. These new CRISPR systems have more precise and diverse DNA editing capabilities compared to traditional genome editing tools. They can achieve things that traditional tools cannot, such as activating genes.
Marianne Carlon, a lung disease expert at the Respiratory and Thoracic Surgery Laboratory of the Catholic University of Leuven in Belgium, believes that the regulatory approval of CRISPR/Cas9 lays the foundation for the next generation of genome editing technology.01
New Applications of Single Base Editing Technology
Single base editing is a gene editing tool developed by the team of David Liu from Harvard University in 2017. Single base editing technology was named one of the “Top 10 Breakthroughs in Science of the Year” that year, and David Liu also made it to the “Top 10 Nature Figures of the Year” in 2017.

Single base editing can be applied to treat cystic fibrosis mutations, which can damage the lungs and digestive system, a problem that traditional CRISPR/Cas9 cannot solve. Gottesdiener believes that the reason why CRISPR/Cas9 technology cannot solve it is because “CRISPR is better at destroying than repairing”.
Currently, Carlon is researching how to use single base editing to treat cystic fibrosis. This editing technique can change individual DNA letters or bases, such as changing A to G or C to T. It utilizes the Cas9 enzyme in the initial CRISPR system to accurately locate these point mutations.

It has been 7 years since single base editing was first reported, and scientists have found ways to reduce unnecessary DNA changes. They have also reduced the size of editing tools, making them easier to enter cells.
Now, single base editing therapy has been applied in early clinical trials, including the treatment of high cholesterol and certain types of leukemia.
However, despite its high precision, this technology has some drawbacks: it can only alter specific DNA sequences and cannot insert new DNA fragments into the genome.
Leading gene editing is gaining momentum
In 2019, a new CRISPR system called Prime editing emerged. This gene editing technology was also developed by the team of David Liu (Liu Ruqian).
Lead editing can achieve arbitrary base substitution, DNA fragment insertion, and deletion. Compared to single base editing, it is more flexible and can target and modify almost any site in the genome.
But on the contrary, it is also more complex. Carlon said, “It has diversity, but it also adds some operational challenges.”.

Since 2019, scientists have improved the design of enzymes to make lead editing more efficient. At the same time, other improvement measures should be taken to prevent errors caused by the natural DNA repair mechanism of cells.
The Prime Medicine program is expected to apply to the FDA next year to initiate a pilot editing therapy clinical trial targeting chronic granulomatosis, a hereditary immune disorder.
At the same time, researchers are attempting to explore new areas of technology by inserting increasingly large DNA fragments into specific locations within the genome. This paves the way for the replacement of the entire gene.
Omar Abudayyeh, a biological engineer at the Massachusetts Institute of Technology Cambridge, said that this will make it easier to treat hereditary diseases such as cystic fibrosis, as this disease may be caused by multiple different mutations in a specific gene. This means that one day, we can treat it by replacing defective gene copies, rather than designing different treatment plans for each mutation.
The potential of epigenome editing
In addition to altering the sequence of genes themselves, the CRISPR system can also regulate gene activity by modifying the epigenome, including chemical modifications of DNA.
In 2021, scientists from the Massachusetts Institute of Technology (MIT) and the University of California, San Francisco (UCSF) jointly developed a new type of epigenetic editing called CRISPRoff. This editor can instantly achieve persistent, heritable, and reversible DNA methylation modifications and gene transcription regulation.

However, unlike single base editing, the development speed of epigenome technology is relatively slow. This is partly because scientists once believed that epigenomic editing performed during cell division would be eliminated. Derek Jantz, Chief Scientific Officer of Tune Therapeutics, explained, “This is a common misconception… Epigenetics has a lasting impact on genes.”
In May of this year, Tune’s scientists conducted an experiment that successfully shut down the PCSK9 gene in non-human primates without altering the core structure of DNA. They used a technique to add chemical markers called methyl groups to DNA, which can affect the way genes work. According to Jantz, this impact has been ongoing for at least 11 months.
Jantz pointed out that this long-term effect may give epigenome editing an advantage in certain RNA drugs that require regular re administration. Meanwhile, this treatment method does not alter the DNA itself, which helps alleviate regulatory concerns about the safety of CRISPR/Cas9 treatment.
A deeper understanding of the epigenome can help us develop more effective treatment methods, and even address diseases that other forms of CRISPR editing cannot handle. For example, Tune plans to use epigenome editing methods to treat hepatitis B virus infection. They hope to effectively control this infection by affecting the viral DNA inside cells, even when the virus remains latent after antiviral treatment.
Although these applications differ from the CRISPR/Cas9 editing used in the first approved CRISPR drug, regulatory approval demonstrates that CRISPR based editing is a feasible method for treating diseases.
“The approval of gene editing technology is a significant event, and similar technologies may enter a stage of rapid development in the future.”