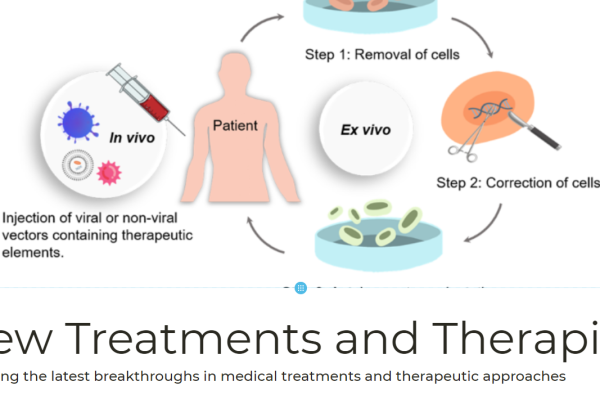
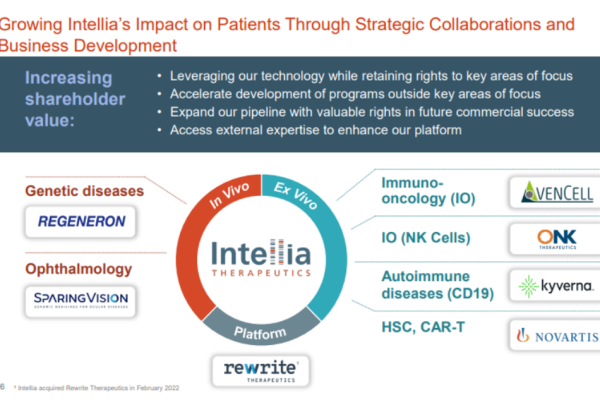
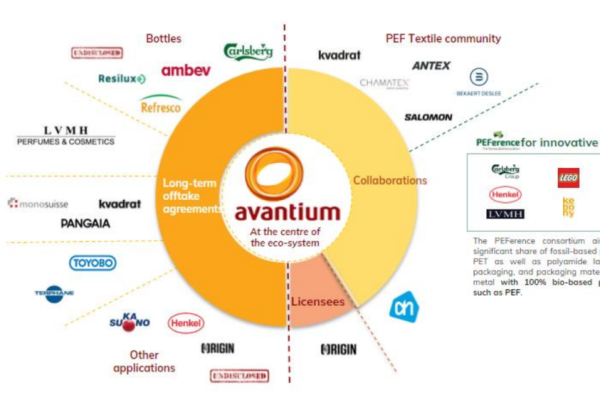
The world’s first AI designed and open-source CRISPR gene editor has successfully edited human DNA
CRISPR gene editing is widely recognized as the most significant and groundbreaking breakthrough in life science since the 21st century. It was officially born in 2012 and was recognized by the Nobel Prize in just 8 years. At the end of last year, the first CRISPR based gene editing therapy was approved by the FDA…