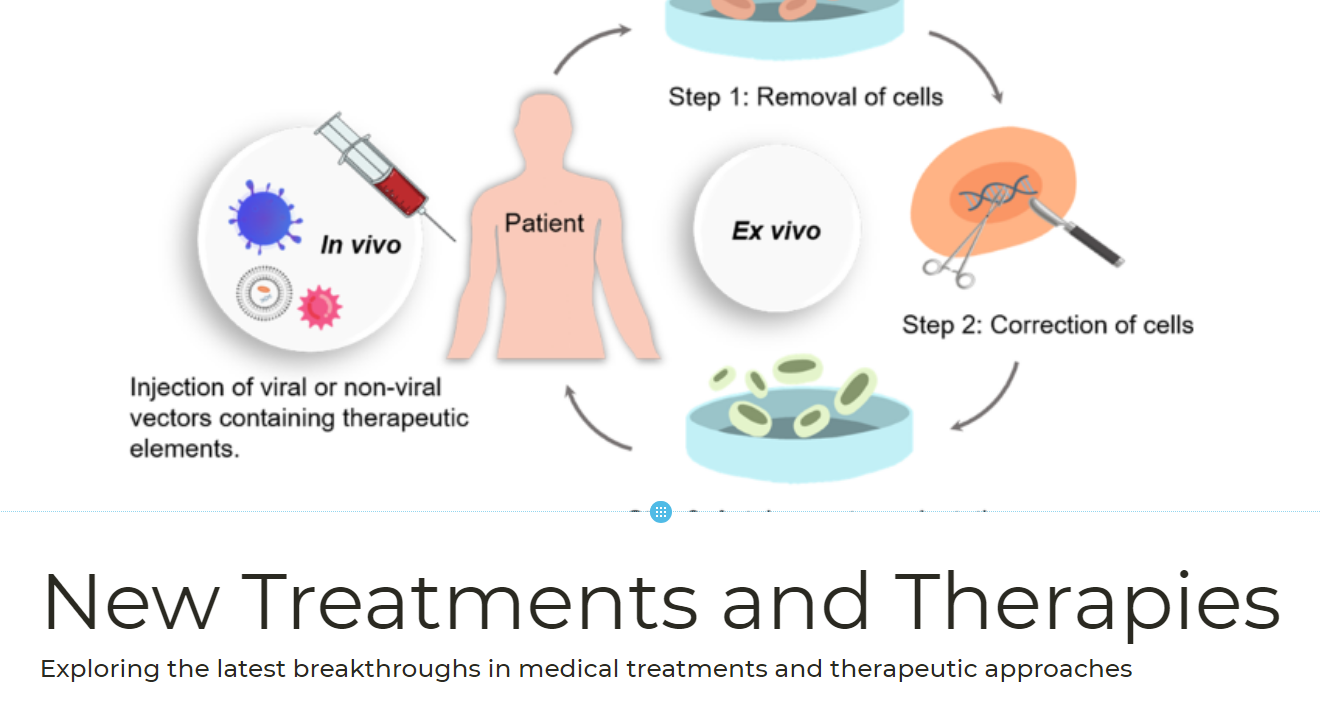
Since the intensive progress in the field of gene editing therapy was released, it has to some extent boosted confidence in the industry. The attitude and actions of the FDA are key factors in promoting the commercialization of gene editing therapy.
Since Feng Zhang first demonstrated in 2013 that CRISPR-SpCas9 can successfully perform gene editing in human cells, the field has rapidly developed and blossomed in multiple directions. In the past 10 years, related editing tools and technologies based on Cas9 have rapidly iterated, such as epigenetic tools, base editing, prime editing, PASTE, CAST, etc. A single tool has presented multifunctionality. It can be said that the editing tasks that SpCas9 could perform before have become more compatible and accurate with later tools.
Previously, some people were worried that the technology iteration in this field was so fast, but the drug development cycle was also very long, which would result in the 1.0 version of the technology not yet being commercialized, and higher versions such as 2.0>3.0 having already caught up. By then, it seemed like they had ended up being trapped in their own fate.
At present, this situation is indeed happening and has already occurred. For example, certain targeted therapies can be achieved through knockout using Cas9; Later base editing was also able to achieve this goal and could also repair specific amino acids; Later on, Prime Editing was not only able to perform tasks that Base Editing could do, but could also complete repair tasks for more bases at once than Base Editing.
| Catalog Number | Product Name | Product Size | Applications | Price |
|---|---|---|---|---|
| GE0001L | Cas9-NLS | 400 pmol | Random Mutagenesis | Online Inquiry |
| GE0001X | Cas9-NLS | 2,000 pmol | Random Mutagenesis | Online Inquiry |
| GE0002S | CD® Cas12a | 70 pmol | Random Mutagenesis | Online Inquiry |
| GE0002X | CD® Cas12a | 2,000 pmol | Random Mutagenesis | Online Inquiry |
| GE0003S | CD® Cas9 Nickase | 70 pmol | Random Mutagenesis | Online Inquiry |
| GE0003L | CD® Cas9 Nickase | 400 pmol | Random Mutagenesis | Online Inquiry |
| GE0004S | CD® Cas9 Nuclease | 70 pmol | Random Mutagenesis | Online Inquiry |
| GE0004L | CD® Cas9 Nuclease | 400 pmol | Random Mutagenesis | Online Inquiry |
| GE0004X | CD® Cas9 Nuclease | 2,000 pmol | Random Mutagenesis | Online Inquiry |
| GE0005 | CRISPR Nuclease mRNA | 15 μg | Insertion of target sequence into the Cas9-sgRNA constructs. | Online Inquiry |
| GE0006 | gRNA Synthesis Kit | 20 Reactions | Random Mutagenesis | Online Inquiry |
| GE0007S | Monarch RNA Cleanup Kit | 10 Preps | Random Mutagenesis | Online Inquiry |
| GE0007L | Monarch RNA Cleanup Kit | 100 Preps | Random Mutagenesis | Online Inquiry |
| GE0008S | sgRNA Synthesis Kit | 10 Reactions | Random Mutagenesis | Online Inquiry |
| GE0008L | sgRNA Synthesis Kit | 20 Reactions | Random Mutagenesis | Online Inquiry |
| GE0009S | SNAP-tagged dCas9 | 70 pmol | Random Mutagenesis | Online Inquiry |
| GE0009L | SNAP-tagged dCas9 | 400 pmol | Random Mutagenesis | Online Inquiry |
| GE0010S | Staphylococcus aureus Cas9 | 70 pmol | Random Mutagenesis | Online Inquiry |
| GE0010L | Staphylococcus aureus Cas9 | 400 pmol | Random Mutagenesis | Online Inquiry |
Finally, this was reflected in Intellia Tx’s Q3 2023 quarterly report released last week, which can be considered an official reflection of the industry. Intellia’s earliest in vivo gene editing therapy pipeline used Cas9 targeting to generate indels for target knockout strategy. In the Q3 quarterly report, for the first time, the official announced the termination of the in vivo gene knockout strategy for the original project NTLA-2003 and the shift towards using the company’s DNA writing technology to advance the project. Of course, the specific details are unknown, but most analysts suggest that this will lead to more recognition and demand for technologies similar to Prime Editing.
Perhaps this is the future of gene editing therapy technology.
On November 12, 2023, Verve Tx will release the first clinical trial data based on base editing, which is another landmark moment for us to wait and see.
In the movie “Oppenheimer,” there is a line that Strauss asks Oppenheimer, “Albert is the greatest scientist of his time, why not let him join?” Oppenheimer says, “No, that’s his time…”
Related Services
What are the highlights of Intellia Tx’s quarterly report?
On November 9th Eastern Time, gene editing therapy company Intellia Tx publicly released its third quarter report for 2023. According to the highlights disclosed on its official website:

The FDA has approved the IND application for NTLA-2001, the first in vivo CRISPR candidate drug to enter later clinical development, and is expected to initiate a critical phase 3 clinical trial for patients with amyloidosis associated with thyroxine converting protein (ATTR) and cardiomyopathy by the end of the year; Currently, clinical data from over 60 patients with ATTR amyloidosis undergoing NTLA-2001 treatment show that after a single dose of NTLA-2001 treatment, serum TTR continues to decrease in depth and duration, with a median decrease of over 90%;
In the fourth quarter of 2023, patients who are expected to complete the Phase 2 study of NTLA-2002 for the treatment of hereditary angioedema (HAE) will be enrolled;
In the first quarter of 2024, it is planned to submit a clinical trial application for NTLA-3001, a candidate drug under development for in vivo insertion editing, for treatment α- 1. Lung diseases related to anti trypsin deficiency (AATD);
Intellia and Regenerative Element are expanding their research collaboration to develop CRISPR based in vivo gene editing therapies for neurological and muscular diseases;
In the third quarter of 2023, the cash reserve was $990 million, indicating a strong financial situation.

In vivo gene editing pipeline status update:
Amyloidosis of thyroxine converting protein (ATTR)
NTLA-2001 type: NTLA-2001 is an in vivo, systemic delivery, CRISPR based research therapy aimed at inactivating the TTR gene in liver cells, thereby preventing the production of transthyroxine protein (TTR) protein, used for the treatment of ATTR amyloidosis. NTLA-2001 provides the possibility of preventing and reversing diseases by promoting deep, sustained, and potentially lifelong reduction of TTR protein after a single administration.

Among all patients receiving a dose of 0.3 mg/kg or higher (n=62), the median serum TTR decreased by 91% on day 28, and the median absolute residual serum TTR concentration was 17 μ G/mL. NTLA-2001 showed overall good tolerability in all patients and at all test dose levels, with most adverse events being mild in severity.

The company is actively preparing for the Global Critical Phase 3 study of NTLA-2001, including discussions with regulatory agencies. In October, the US Food and Drug Administration (FDA) approved the Phase 3 Investigative New Drug (IND) application for NTLA-2001. The Magnitude Phase 3 trial is a randomized, double-blind, placebo-controlled study aimed at evaluating the efficacy and safety of NTLA-2001 in approximately 765 ATTR-CM patients
The efficacy and safety of the treatment. The main endpoint of this study is the composite endpoint of cardiovascular (CV) related mortality and CV related events. Patients will be randomly assigned 2:1 NTLA-2001: placebo, with a single infusion of 55 mg NTLA-2001. The company expects to initiate the study before the end of the year and begin patient administration in early 2024.
Hereditary angioedema (HAE)
NTLA-2002: NTLA-2002 is a systemic CRISPR research therapy in vivo. NTLA-2002 aims to knock out the KLKB1 gene in the liver, which may permanently reduce the total protein and activity of plasma kallikrein, a key mediator of HAE. This research method aims to prevent the onset of HAE in patients by continuously reducing plasma kallikrein activity after a single administration. It also aims to eliminate the significant therapeutic burden associated with currently available HAE therapies. NTLA-2002 is being evaluated in a phase 1/2 study targeting adults with type I or type II HAE.

In October, Intellia announced that the European Medicines Agency (EMA) had granted NTLA-2002 Priority Medicines (PRIME) approval for the treatment of HAE. After identifying all patients, Intellia is expected to complete the patient enrollment for Phase 2 clinical trials in the fourth quarter of 2023. The Intellia program initiated a globally critical Phase 3 study, including American patients, as early as the third quarter of 2024, subject to regulatory feedback.

α- 1. Antitrypsin deficiency (AATD)
NTLA-3001 is used for AATD related lung diseases:

NTLA-3001 is the first CRISPR mediated in vivo targeted gene insertion development candidate drug for the treatment of AATD related lung diseases. It aims to precisely insert encoding α- A healthy copy of the SERPINA1 gene of the anti trypsin (A1AT) protein and the possibility of restoring permanent expression of the functional A1AT protein to therapeutic levels after a single administration. This method aims to improve the prognosis of patients, including in severe cases without the need for weekly intravenous A1AT enhancement therapy or lung transplantation. Intellia plans to submit a clinical trial application in the first quarter of 2024 to initiate the first human clinical phase 1 study of NTLA-3001.

NTLA-2003 is used for AATD related liver disease: NTLA-2003 is an in vivo knockout candidate drug for the treatment of AATD related liver disease. It aims to inactivate the SERPINA1 gene responsible for producing abnormal A1AT protein in the liver. Based on resource prioritization, the company is discontinuing further IND support activities for NTLA-2003 to utilize the company’s DNA writing technology to advance the AATD research phase plan.
In February 2022, Intellia announced the acquisition of Rewrite Tx, a California based company, to enhance its platform strength in gene editing and writing technology.