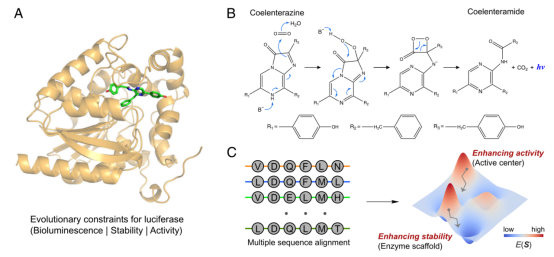
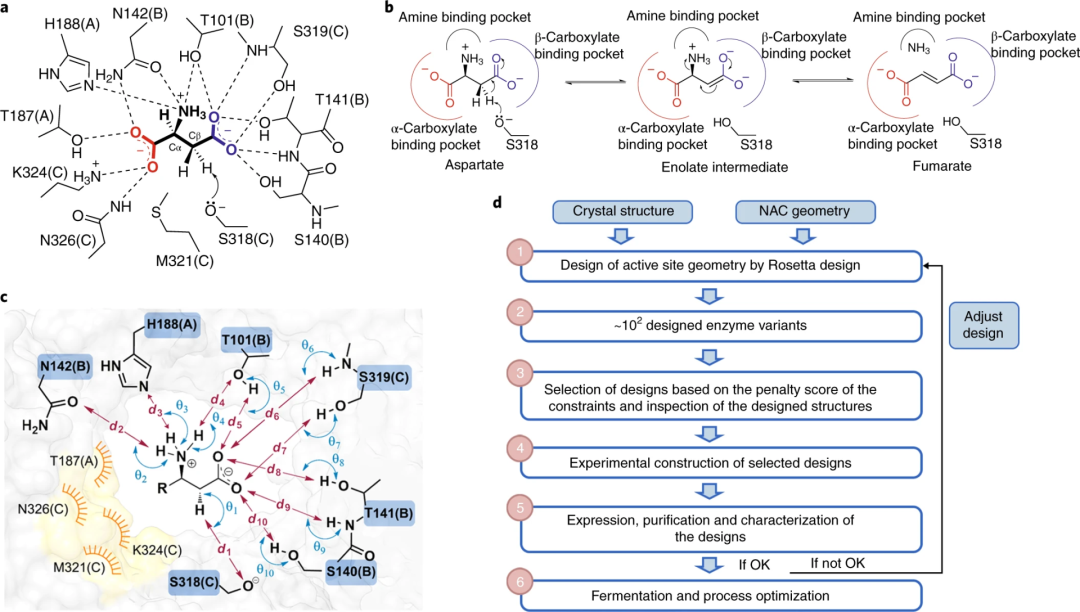
In enzyme engineering, life and death selection allows for the rapid and direct identification of efficient biocatalysts from a large library. However, the evolution of biocatalysts (related to industry) based on life and death selection is challenging as they require highly reliable strategies to artificially link their activity to host survival.
Recently, Clemens Mayer from the University of Groningen in the Netherlands published a preprint of “A robust life or death selection platform for enzyme evolution” in BioRxiv. The authors constructed a non classical amino acid dependent Escherichia coli platform, where the precursor of the non classical amino acid, N-carbamylated 3-nitro-L-tyrosine, was catalyzed to produce 3-nitro-L-tyrosine by an L-N-carbamyl hydrolase mutant from the Chinese rhizobia of alfalfa. And combine it with engineering using an orthogonal translation system that suppresses the termination of codons within the reading frame β- In the case of exogenous addition of carboxybenzyllin to lactamases, the growth rate of Escherichia coli cells is related to the activity of carbamoyl hydrolase mutants (producing 3-nitro-L-tyrosine). Subsequently, the author designed two enzyme mutation libraries, Lib_ 2N and Lib_ 4N, by randomly selecting two or four residues arranged on the binding pockets of carbamoyl hydrolase. Specifically, Lib_ 2N targets both Leu217 and Phe329 genes (due to their high synergistic effect), LiB_ 4N also includes adjacent residues Gln215 and Gly327, and its amino acid side chains also extend towards the binding pocket. With a sufficiently diverse mutation library, the author further identified highly active mutants by increasing the concentration of carbenicillin and increasing screening pressure. Finally, the initial reaction rate of the most active mutant towards N-aminoacylated 3-nitro-L-tyrosine was 0.61/s, exceeding the wild-type activity by 7760 times.

Selecting improved variants from heavily-skewed populations
Recommended Services and Products
 |
One-stop Enzyme Discovery and Design ServicesOur One-stop Enzyme Discovery and Design Services are tailored to accelerate your research and development efforts, providing you with efficient and effective solutions for enzyme discovery, optimization, and production. |