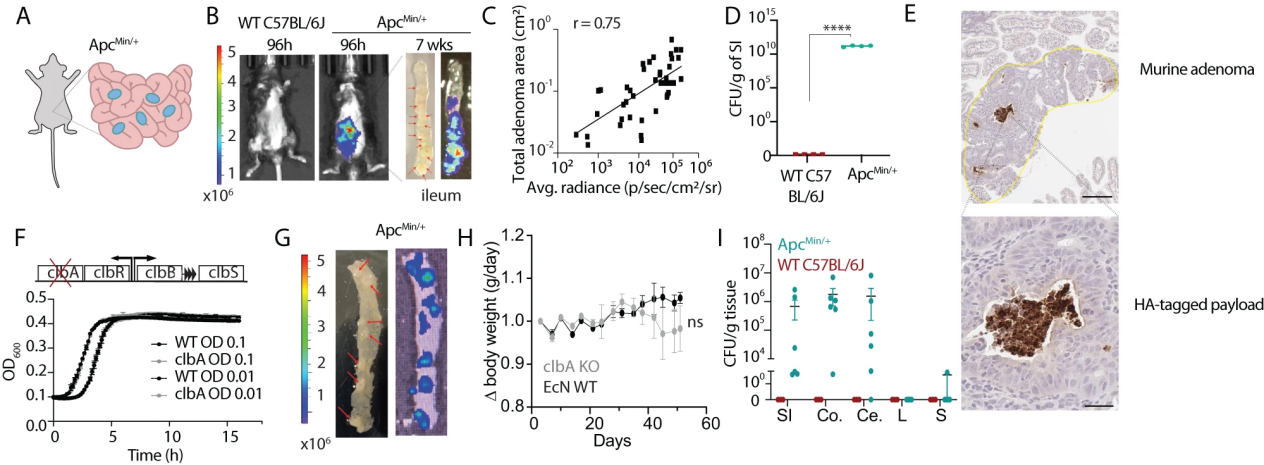
High precision bioengineering and synthetic biology require fine-tuning of gene expression at the transcriptional and post transcriptional levels, and gene transcription itself is strictly regulated by promoters and terminators. Promoters include core promoters and distal promoters (Figure 1), which can determine the practice, organization, and cellular level of gene expression. The terminator is inserted into the gene to terminate transcription and affects the mRNA level after transcription, such as 3 ‘terminal processing, stability, translation efficiency, and the efficiency of cytoplasmic output. Therefore, in high-precision plant bioengineering, determining the optimal combination of promoters and terminators is of crucial importance for efficient transcriptional transgenes.

Figure 1 Locations of the cis regulatory elements (CREs) and core promoter motifs CCAAT, Y patch, TATA box, and TSS/Inr within discot and monocot promoters
On July 7, 2023, BioDesign Research published an online review article titled Plant Promoters and Terminators for High Precision Bioengineering by Emily G. Brooks and others from North Carolina State University. The author provides a detailed introduction to the characteristics of promoters and terminators in plant bioengineering, and provides principles for the selection and combination of the two in plant bioengineering.
The performance of the core promoter depends on its nucleotide sequence, position, copy number, and chromatin structure. By generating a random core promoter with an average nucleotide frequency similar to that of Arabidopsis or corn and adding TATA box, Inr, and Y patch motifs for plant core promoter engineering, it was found that the addition of three additional motifs significantly enhanced the activity of the core promoter. Among them, TATA box increased the intensity the most. This indicates that reasonable design and construction of plant core promoters with different intensities can be achieved by inserting core promoter motifs into appropriate nucleotide backgrounds. At the same time, plant promoters also use the 5 ‘untranslated region and its leading introns to affect post transcriptional mRNA levels and gene function during gene expression. There are also many cis regulatory elements in front of the core promoter, widely distributed in the proximal and distal promoter regions. Many of these elements serve as specific regulatory factors for gene expression, stimulating (enhancers) or inhibiting (suppressors) the basal expression levels assigned by the core promoter.
The termination of gene expression in plants relies on the synergistic action of three cis termination elements, including proximal upstream element (NUE), distal upstream element (FUE), and cleavage site (CS). Plant terminators can affect gene expression levels by determining the cleavage sites of mRNA and the length of transcripts. Research has also shown that linking two terminators together can effectively enhance the expression of transgenic genes. In addition to directly affecting mRNA transcription levels, the matrix attachment region in the terminator can also alter the chromatin structure and conformation by binding to the protein nuclear matrix, thereby playing a role in epigenetic regulatory sequences.
Given the important role of promoters and terminators in regulating the expression intensity and spatiotemporal expression characteristics of genes, it is crucial to use promoters and terminators to achieve precise regulation of genetic circuits in complex plant system bioengineering. The author then highlighted some representative examples on plant transformation, plant metabolic engineering, plant-based biosensing, and genome editing, covering multiple technical aspects including expression intensity, expression patterns, terminator and promoter configurations.
So far, approximately 8000 plant promoter sequences have been identified through transcriptomics and genomics and recorded in the plant promoter database, but only a few of these elements have been characterized and validated in plants. Therefore, there is an urgent need to increase the number of promoters and terminators for functional characterization as standard biological components. The author summarized the standard characterization methods for characterizing the characteristics of promoters and terminators. These components can be systematically evaluated from four aspects: temporal expression patterns, spatial expression patterns, environmental response, and cross species variation (Figure 2).

Figure 2 Aspects of promoter and terminator evaluation for plant biosignature
The verification methods for promoters and terminators are usually transient expression methods and stable expression methods. The former typically uses microscopy, RT qPCR, and ddPCR for functional characterization, while the latter typically uses GFP reporter genes to verify the performance of promoters and terminators. In addition to the common reporter system, the genetic circuit for verifying promoter activity can also use a dual reporter gene system to eliminate differences in transmission efficiency and regulate the expression of reporter genes (Figure 3).

Figure 3 The technologies for benchmarking promoters and terminators in plants
The author systematically reviewed the application of promoters and terminators in fine regulation of gene expression in plant bioengineering, as well as standardized methods for characterizing plant promoters and terminators. This will provide valuable insights for the characterization, planning, and construction of genetic circuits and pathways in subsequent plant biological design.