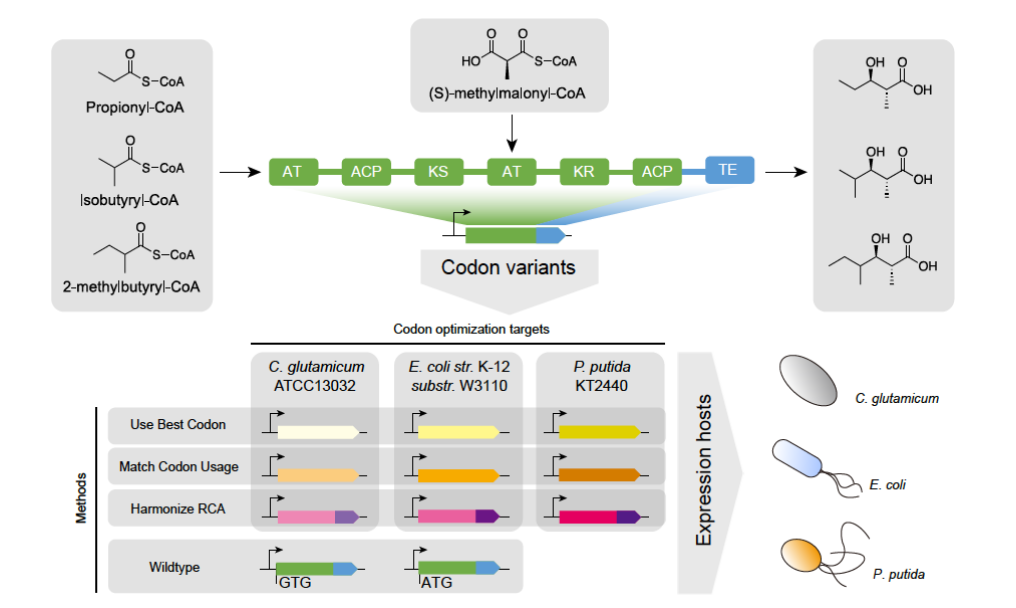
The drawbacks of traditional microbial fermentation processes include the risk of contamination by harmful microorganisms, the inhibitory effect of high concentration carbon sources in the culture medium, microbial thermal sensitivity, and instability. And low yield and productivity, low substrate utilization efficiency, production of by-products and secondary metabolites, or high downstream processing costs. In order to overcome these problems, attempts have been made through cell-free systems (CFSs). However, traditional CFSs have been developed from crude cell extracts and have not been further optimized, lacking stable enzymes and cofactors. They have the drawbacks of thermal instability, enzyme inactivation, high cost, and non reusability. This also includes issues such as feedback inhibition, cofactor degradation, depletion, and loss of intermediates in multi enzyme catalytic reactions. This requires the development of advanced and stable CFSs for large-scale production of different materials and industrial applications.
The latest research in synthetic biology and metabolic engineering provides a launching pad for the development of cellless synthesis systems (SyCFSs), which implement advanced synthetic biology tools for in vitro metabolic engineering. Unlike traditional CFSs, SyCFSs perform complex biochemical reactions without cell wall/membrane barriers and biological energy limitations. They follow the principles of synthetic biology, such as using oligonucleotides as primers for PCR amplification of DNA synthesis, RNA for in vitro transcription, and peptides synthesized through in vitro transcription and translation (TX-TL). SyCFS is composed of many purified enzymes and cofactors, forming a metabolic pathway for producing target products with expected characteristics and high yield; It has the advantages of improving product yield, small reactor volume, wide reaction conditions, fast reaction speed and short cycle for multi enzyme reactions with fewer operations; And non natural biological products formed through the recombinant TX-TL system are easy to separate. Biohydrogen, bioethanol, protein scaffolds, and cell-free proteins (including complex proteins, toxic proteins, membrane proteins, functional antibodies, and non natural amino acids (UNAAs)) are all produced through this technology. The recently developed mathematical models and computational tools, with the help of the Cellless Protein Synthesis System (CFPS), provide the foundation for the fine manufacturing of the next generation of artificial cells. A typical CFPS, its components and its application in designing the minimum unit model are shown in Figure 1.

 Fig1 Cell Free Protein Synthesis System
Fig1 Cell Free Protein Synthesis System
Although the potential benefits of CFSs are obvious, they are relatively new and in the early stages of development. Therefore, extensive research is still needed to commercialize it. However, due to the high price of auxiliary factors as energy driven biochemical reactions, CFSs face economic feasibility issues. The latest research in biological processes and metabolic engineering provides a foundation for designing cell-free synthesis pathways using low-cost and stable biomimetic cofactor mimics. By carefully designing and regenerating auxiliary factors, optimizing enzyme loading and activity, using high substrate concentration, and increasing reaction temperature, the efficiency of CFSs can be improved. So far, cofactor regeneration through molecular metabolism of glucose, phosphoenolpyruvate (PEP), glucose-6-phosphate (G6P), phosphocreatine (CP), phosphoacetyl (AP), and pyruvate has been confirmed.
The difference between CFS and traditional microbial fermentation is that enzymes representing key components of the system cannot replicate, while microbial cells can replicate themselves when providing nutrition and appropriate growth conditions. In addition, it also has the advantages of directly entering complex biological processes, adding new ingredients at a reasonable price, providing a controllable chemical environment, active regulation, and simple and fast sampling. Can minimize the system’s response to environmental stimuli and allocate resources specifically for target product production to improve efficiency.
Introduction to Cellless System Technology
1. A cell-free system based on crude cell extract
Traditional CFS is composed of crude cell extracts and is obtained by disrupting extracellular barriers such as cell walls or membranes, or both. The overall efficacy of such processes is determined by the combined action of all enzymes. Overall, there are two methods available for developing cell-free systems based on crude extracts (CE-CFS): direct (one-step) and indirect (two-step) development strategies
 Fig2 Cell-free system based on crude extract
Fig2 Cell-free system based on crude extract
In the direct method, the required product is synthesized in vitro using crude cell extracts containing equilibrium cofactors and mixed enzymes. The absence of membrane barriers ensures that metabolic reactions cannot or are difficult to achieve in the body under other conditions, but this method can produce by-products. Therefore, downstream processing is required to separate the required products. In indirect methods, a two-step approach is adopted, which involves blocking certain metabolic pathways in the target organism in the body through metabolic or genetic engineering strategies. This targeted utilization requires complete information about specific metabolic pathways. In addition to the absence of membrane barriers, cell-free methods promote the supplementation of substrates and cofactors, minimize the impact of toxic compounds on microbial cells, and provide a pathway for direct contact with synthetic products. The ce-cfs based system provides detailed information on cell metabolism and energy generation during CFPS, and provides a deep understanding of the regulation of carbon flux in the metabolic process in the body, providing a unique platform for studying complex biological systems. CFSs have been used for several applications, such as Welch and Scopes developing a recombinant CFS for ethanol production. Subsequently, Shimizu et al. developed a reconstructed CFS from the factors required for protein synthesis. CE-CFS has also received great attention in high-throughput screening, proteomics, UNAA binding, and personalized healthcare. By mixing template DNA with amino acids, nucleotides, energy sources, and cell extracts containing ribosomes and cellular enzymes, combined with TX-TL reactions, it has significant applications in protein synthesis. In another study, CE-CFS based on G.hansenii was developed for the production of cellulose, and the synthesized cellulose had much better physical and mechanical properties than microbial cellulose. Ullah et al. also developed a one pot strategy for in-situ impregnation of titanium dioxide nanoparticles into a cellulose matrix based on G.hansenii’s CE-CFS (Figure 3). In the synthesis process of nanocomposites, suspended titanium dioxide nanoparticles interact with growing cellulose and are trapped in the matrix through hydrogen bonds, causing some nanoparticles to be physically fixed between fibers, bypassing the toxicity of nanoparticles to microbial cells; Secondly, it ensures the efficient absorption and uniform distribution of nanoparticles in nanocomposites.
However, the large-scale industrial application of traditional CE-CFS is often hindered by its limited lifespan. The cellless production of biological commodities involves the continuous supply of substrates. However, so far, active control of substrate addition based on actual reagent concentration has not been achieved. In order to address these issues, with the assistance of a comprehensive energy regeneration system, efforts have been made to develop more advanced cell-free bioreactors, as well as corresponding control systems and feedback devices, to control the feeding rate of substrates.
2. SyCFS in vitro engineering using principles of synthetic biology
Compared to traditional CFS extracted from cell extracts, the synthetic cellless system (SyCFS) is only composed of specific enzymes required to synthesize the required proteins. The design of SyCFS must consider several parameters, such as the balance of auxiliary factors, process thermodynamics, reaction equilibrium, yield, product separation and separation, and total capital cost. One pot sycfs is often trapped by several issues, such as feedback inhibition of products or intermediates, diffusion loss of reaction intermediates, consumption and inability to regenerate of cofactors, and the presence of impurities in by-products. Therefore, the main focus of designing SyCFS is to overcome these obstacles and achieve maximum production at the lowest cost. In the process of developing SyCFS, factors such as stable enzyme selection and immobilization methods, stability and regeneration of auxiliary factors, in situ separation of products, and reactor engineering must be considered.
Design of a synthetic cell-free system. Zhang et al. classified the complexity of biochemical conversion processes into (1) single enzyme catalysis, (2) multi enzyme catalysis, (3) CFS, and (4) whole cell fermentation based on their increasing complexity. The complexity of this biochemical transformation biological process can be described in terms of its machinery, preparation methods, self-replication ability, reaction complexity, downstream processing requirements, product purity, yield, and other aspects. The presence of a single enzyme is beneficial for producing only the required products without producing by-products, requiring minimal downstream processing. A good example of single enzyme catalysis is the use of DNA polymerase to amplify DNA through PCR. In contrast, multi enzyme catalysis can involve relatively complex chemical reaction sequences, where each step is mediated by a specific enzyme, and the activity of each enzyme is regulated by the presence or absence of specific cofactors. In vitro CFS may be assembled by multiple enzymes and/or cofactors without an outer membrane barrier. Finally, whole cell fermentation involves the conversion of substrates into different products in the body through interconnected metabolic pathways, forming a complex metabolic system. There are several differences between CFS and whole cell fermentation: (1) living cells can replicate, but enzymes cannot; (2) Most of the available substrates are used for the feeding, growth, and proliferation of living cells, while in CFS they are only used for product formation; (3) CFS has a high yield, efficacy, and yield, which is an inherent limitation of the cellular system. Zhang suggests dividing the SyPaB system into five steps: (1) pathway reconstruction, (2) enzyme selection, (3) enzyme engineering, (4) enzyme production, and (5) process engineering. However, considering that some enzymes may require additional structural modifications to obtain the required functions, or are produced by engineered strains and further purified, we suggest dividing the “enzyme selection” step into two steps: “enzyme selection” and “enzyme separation and purification”. And additional steps involving in vitro metabolic engineering were introduced, thus introducing a six step path for successfully developing SyPaB (Figure 3)

Fig3 Synthetic Cellless System (SyCFS)
This synthesis method involves the in vitro assembly of various enzymes and their cofactors, conducting a series of complex biochemical reactions, which is significantly different from cellless biotransformation. The goal of SyPaB’s strategy is to produce low-cost biological products on a large scale, such as biofuels, biopolymers, organic acids, and carbohydrates, while cellless biotransformation is typically used to obtain high-value biological commodities for biomedical applications on a small scale.
The development and production cost of cellless biological manufacturing are one of the main bottlenecks in its scalability and industrialization. It is mainly related to the production and purification of cofactors, enzymes, raw materials, and energy input costs. By improving process efficiency through various strategies, the total cost of cell-free biological manufacturing processes can be minimized.
Due to the development of related fields such as genomics in recent years, CFS has made significant progress and synthesized some useful products through this method. However, cellless biosynthesis still faces many challenges, among which the main obstacle is the lack of stable enzymes and their complexes, as well as expensive cofactors. Therefore, designing SyCFS requires low-cost and stable enzymes, cofactor balance and regeneration systems, biomimetic coenzymes, and low-cost substrates. Enzymes must remain stable, intact, and have catalytic activity within a few weeks in both cell-free and membrane free environments. It also has thermal stability and does not undergo protein hydrolysis under corresponding cell-free production conditions. Therefore, based on the current development of SyCFSs design, it is necessary to further develop control systems to monitor and regulate biological processes, develop various partitioning strategies, optimize molecular circuits and metabolic pathways, and provide energy for system operation. The application of simulation tools, databases, mathematical modeling, and new statistical tools will further promote the development of cellless systems. Finally, combining specific enzymes and cofactors in vitro to reprogram existing or develop advanced synthesis processes is a potential research direction for the next generation of synthetic biology.