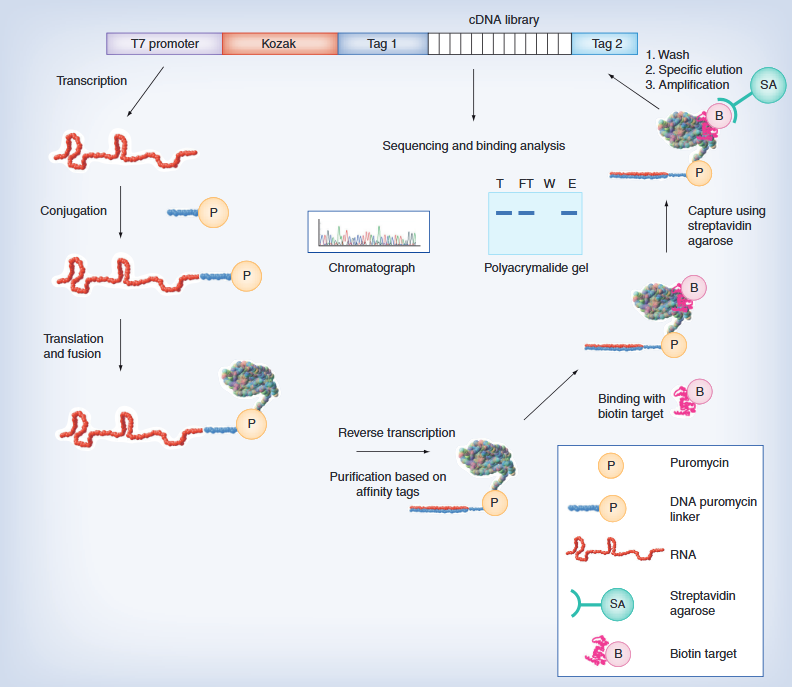
On November 8, 2023, after 15 years, the Synthetic Yeast Genome Project 2.0 (Sc2.0) research team announced a significant milestone: all 16 chromosomes of synthesized yeast, including a newly synthesized tRNA chromosome, have been synthesized, creating a yeast strain containing over 50% synthetic DNA. The relevant achievements have been published in Cell, Molecular Cell, and Cell Genomics, with a total of 10 papers.
Sc2.0 is a global alliance dedicated to synthesizing the entire yeast genome. Led by Dr. Jef Boeke from New York University, involving teams from the UK, the United States, China, Singapore, and other regions.
In order to better manipulate yeast, researchers removed potentially “junk” non coding DNA fragments and repetitive elements, added new DNA fragments to make it easier to distinguish between synthetic and natural genes, and introduced a built-in diversity generator called “SCRaMbLE” that can disrupt the sequence of genes within and between chromosomes.
To increase the stability of the genome, the research team also removed many genes encoding transfer RNA (tRNA) and relocated them onto a completely new “new chromosome” composed entirely of tRNA genes.
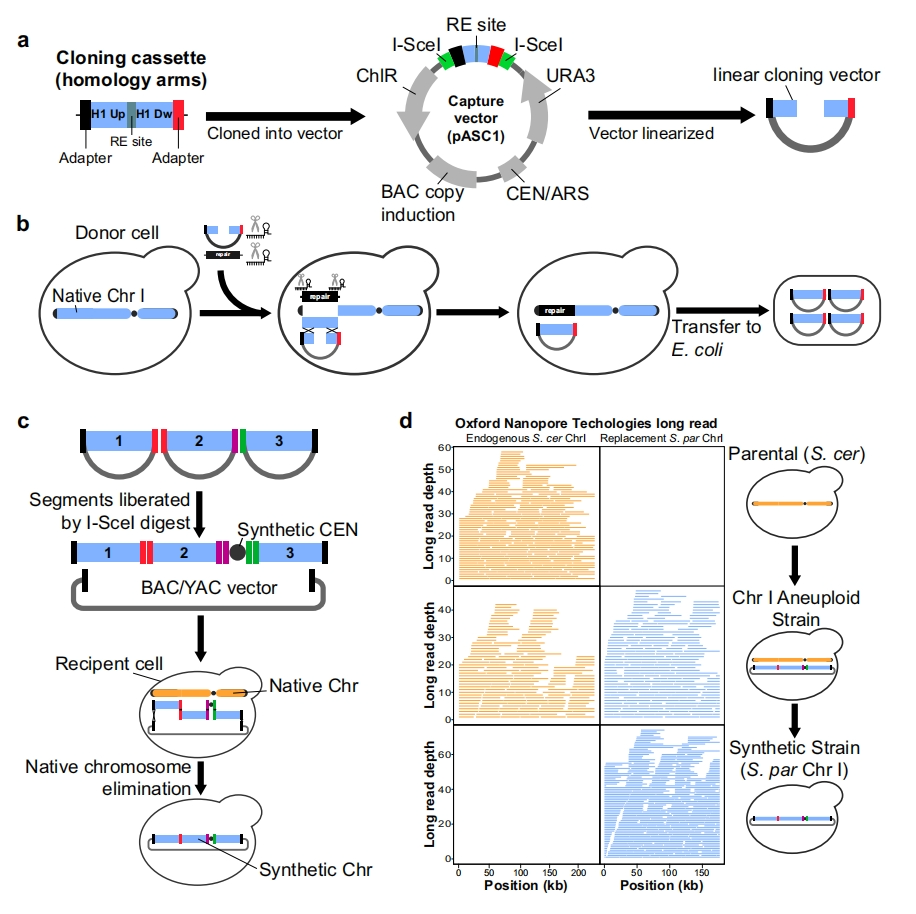
Due to the yeast genome being composed of 16 chromosomes, researchers began assembling each chromosome independently, creating 16 partially synthesized yeast strains, each containing 15 natural chromosomes and 1 synthetic chromosome. The next challenge is to start combining these synthetic chromosomes into a yeast cell.
To this end, Boeke’s team used a method similar to Mendelian peas: hybridizing different synthetic yeast strains and then searching for individuals carrying two types of synthetic chromosomes in their offspring. The research team gradually integrated all synthetic chromosomes -6 complete chromosomes and 1 chromosome arm – into one cell. The final yeast strain obtained has a synthesis rate of over 31%, normal morphology, and only slight growth defects compared to wild-type yeast.
To more effectively transfer specific chromosomes between yeast strains, researchers have developed a new method called chromosome substitution. They used chromosome substitution to transfer a newly synthesized chromosome (chromosome IV, the largest of all synthesized chromosomes), resulting in a yeast cell with 7.5 synthesized chromosomes that could survive and replicate.

Yeast cells containing 7.5 synthetic chromosomes can sprout normally and divide into two cells (source: Cell)
When synthetic chromosomes were integrated into a single yeast strain, the research team discovered several genetic defects that were not visible in yeast strains carrying only one synthetic chromosome. Some of these errors are simply due to the superposition of many small defects in the genome, while others involve genetic interactions between genes on different synthetic chromosomes. By using CRISPR/Cas9 based methods, researchers were able to fix several of these errors and improve the synthesis of yeast.
We have demonstrated that almost half of the synthetic genome can be integrated, and the resulting yeast strain still has good adaptability, “Boeke said. Next, researchers will integrate the remaining synthetic chromosomes, which are expected to be completed next year.
Related recommendations
Yeast Strain Modification
Yeast Cell Surface Engineering
Pichia pastoris Glycoengineering