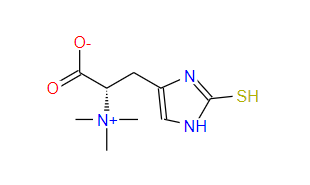
The unique sulfur-based chemical properties of thiols play an important role in the protection of cellular function and components. The body has evolved to rely on highly abundant low molecular weight thiols, such as glutathione (GSH), to maintain redox balance, but also play other important roles, including xenobiotic detoxification and signal transduction. Some of these thiols may also be extracted from the diet, such as the trimethyl betaine derivative of histidine, ergothione (ET). Discovered more than a century ago, interest in this low molecular weight thiol/ketone has reignited, partly due to its unique chemical properties (such as its unusual stability), as well as the recognition of a transporter, the organic cation transporter novel type 1 (OCTN1), responsible for the uptake and accumulation of ET in human tissues in our diet, as well as selective distribution into tissues due to differences in transporter expression. Consistent with recent attention to ET, publications mentioning this compound have shown exponential growth (Figure 1). These include extensive research revealing the relationship between blood ET levels and health and disease, cellular and tissue protective mechanisms, potential therapeutic applications of ET, transporter expression and tissue distribution, biosynthetic mechanisms, and more. This review highlights new insights and discoveries about this extraordinary compound.

Ergothioneine transporter
Organic cation transporter new type 1, Ergothioneine transporter?
In 2005, it was discovered that OCTN1 (encoded by the gene slc22a4) is mainly a transporter of ET, and its transport efficiency is higher than many other related metabolites. Without it (such as knockout animal models), ET is not present in cells and tissues. From the initial concept of OCTN1 primarily serving as an ET transporter, many other studies have shown that OCTN1 is involved in the transport of compounds such as nucleoside, acetylcholine, tetraethylammonium, spermine, L-carnitine, cytarabine, gemcitabine, gabapentin, oxaliplatin, and metformin. However, many of these claims use non-traditional models that are far from physiology. Recently, the use of naturally expressed and overexpressed OCTN1 in human cells has once again confirmed the selective preference of ET as the substrate for OCTN1, supporting the proposal to rename OCTN1 as the ergothione transporter (ET). However, as most groups still use the term OCTN1, we will follow it in this article.
This transporter is responsible for consuming a large amount of ET from the diet and is widely distributed in many tissues (Figure 2). ET has been found in the tissues of most laboratory rats and mice, although their diet contains only trace amounts of ET, indicating effective uptake and retention of this nutrient. The expression of OCTN1 is also present in the proximal renal tubules of rats, which is important for the renal absorption of ET. This may promote the accumulation of ET. In fact, compared to wild-type animals (<10%), OCTN1 gene knockout mice treated with intravenous injection of ET showed a higher urinary excretion rate (>50% of the administered dose). The accumulation ability of ET in the body and its selective distribution in tissues (through differential expression of transporters) indicate that ET plays an important physiological role. However, the OCTN1 gene knockout model (lacking tissue ET) has not shown any substantial dominant phenotype or deficiency syndrome. This may be due to maintaining a steady-state compensatory defense pathway in healthy animals without ET, although the phenotype is more pronounced when these knockout models are under pressure. Due to the large amount of ET present in the diet and its strong retention by the human body, the consequences of its deficiency are difficult to prove in the human body. However, lower blood ET levels are associated with incidence rate of some diseases, including Parkinson’s disease (PD), mild cognitive impairment (MCI), Crohn’s disease (CD) and frailty, while correspondingly higher blood ET levels are associated with lower rates of cardiac metabolic disorders and related mortality and peripheral neuropathy. This is consistent with the view that the lack of ET may increase the risk of disease. Whether the decrease in ET is the cause or consequence of the disease still needs to be determined through in-depth longitudinal research.

Figure 2 | Using normalized consensus RNA expression data from three datasets (genotype tissue expression project, human protein map dataset, and FANTOM5 project), RNA expression of slc22a4 (encoding OCTN1) was obtained in humans. Figures and data from human protein profiles( https://www.proteina Tlas. org/)
Factors affecting the transport of Ergothioneine in the human body
ET can be found in most tissues (if not all), with different accumulation rates due to different expression of transporters. High expression of transporters can be observed in certain cells, so high levels of ET (such as blood cells, bone marrow, eye tissue, brain, etc. Figure 2) may be susceptible to oxidative stress, although other tissues can accumulate high levels of ET. In certain animal disease models, such as fatty liver disease, CD, and chronic kidney disease, the OCTN1 transporter (through transcription activating factors, including RUNXI, Sp1, and NF) κ B) Elevated due to tissue damage. This is considered an adaptive physiological response that can increase the level of ET in damaged tissues, thereby limiting further damage.
Although ET is obtained from diet, studies have shown that the level of ET in tissues is closely related to the expression of OCTN1 or the polymorphism of transporters; The population has many single nucleotide polymorphic variations (SNPs) of OCTN1, which affect ET to motor mechanics. Supporting this viewpoint is that subjects with higher levels of ET in their basal blood also have greater uptake and accumulation of ET after oral administration, possibly due to polymorphism in transporters. However, it is currently unclear whether the polymorphism of OCTN1 (which may explain lower blood ET levels) may make certain individuals susceptible to diseases. The author suggests that this mutation results in up to 50% higher transportation efficiency and a three fold increase in affinity for ET, which is a positive mutation caused by low ET in the diet of these historical populations or high UV exposure of ancient farmers in the region (see section 3.2.6). It is said that the association between OCTN1 polymorphism and IBD or rheumatoid arthritis is still controversial. Longitudinal studies on the SNP of OCTN1 in the population, their blood ET levels, and their relationship with diseases should provide further insights.
Cell localization
Early studies have shown (but not strictly established) that OCTN1 exists in mitochondria, allowing ET to accumulate in this organelle. In fact, ET can protect mitochondrial DNA from oxidative damage caused by hydrogen peroxide or ultraviolet radiation. Since then, there have been few studies on the intracellular localization of OCTN1 and ET. Mitochondrial dysfunction is associated with various diseases, and the therapeutic benefits of ET are believed to be mediated by protecting this organelle. We have recently confirmed that ET can protect mitochondrial morphology in transgenic Drosophila models of PD. Clear evidence of ET uptake and accumulation needs to be found in mitochondria.
Another transporter?
A recent study on an isolated solute carrier (OCTN1 superfamily) SLC22A15 showed that it can transport ET to transfected HEK293 cells, although its transport efficiency is much lower than that of OCTN1. The author believes that the expression of SLC22A15 in the brain is higher than that of OCTN1, which may be important for the deposition of ET in the brain or may translocate in BBB, while the expression level of OCTN1 is still uncertain. However, SLC22A15 does not seem to be involved in ingestion from the diet into the body, as OCTN1 gene knockout mice do not exhibit significant ET levels in the blood and tissues after oral administration. In fact, SLC22A15 is less expressed in the gastrointestinal tract. However, when ET was injected intravenously, the brain levels of OCTN1 knockout and wild-type mice seemed to be comparable, albeit lower. Further research is needed to determine whether SLC22A15 is indeed involved in the brain uptake and accumulation of ET.
Source of Ergothioneine
Cooking angle
ET exists in various foods, but its dietary level is highest in certain mushrooms and spirulina, which produce their own ET. Some studies have investigated the potential health benefits of consuming mushrooms. These factors include increased mushroom intake and dementia, cardiovascular diseases, certain cancers (such as prostate cancer and breast cancer), metabolic syndrome, PD and other neurological diseases, viral infections and many other diseases. Some studies attribute the health benefits of mushrooms to current ET. However, mushrooms also contain many other compounds (such as β- Glucan, polysaccharides, etc.), which may also be beneficial.
The biosynthesis of ET by gut microbiota
The gut microbiota plays a crucial role in human health and diseases. This vast microbial library encodes 100 times more genes than the host, responsible for some essential metabolic functions, including the production of certain vitamins and amino acids. Although animals and humans can only obtain ET from dietary sources, certain bacteria can synthesize ET, including Mycobacterium and Burkholderia. They belong to the Actinobacteria and Proteobacteria respectively. They produce ET from histidine, although through different mechanisms. Transforming Escherichia coli with the egtBD gene (which naturally cannot undergo ET biosynthesis) enables them to produce ET, although the addition of the egtACE gene increases the production of ET in Escherichia coli. An in-depth analysis of bacteria, actinomycetes, cyanobacteria, and proteobacteria present in the normal gut microbiota shows that many of these bacteria appear to have the egtBD gene or their homologues. Therefore, this raises the question of whether the gut microbiota can produce ET. Although there is evidence that this situation has not occurred, it may require re examination using more sensitive and accurate ET analysis methods. If so, will the ET produced by the microbiota contribute to the tissue level of ET, and will changes in the gut microbiota affect the level of ET in the body, as seen in many diseases? This may also be another explanation for the low levels of ET in the blood of many diseases. In fact, with increasing evidence indicating a complex connection between the gut microbiota and the brain, changes in the microbiota leading to a decrease in ET levels in the body may lead to diseases.
So far, no studies have confirmed that the gut microbiota can produce ET, but one study suggests that Lactobacillus reuteri may produce ET in vitro, and Roy’s disease is associated with elevated levels of ET in rat feces. However, the presence of ET in microorganisms is not clear evidence of ET production, as some bacteria can absorb ET from their environment. Isotope labeled precursors need to be used for research or isolation of ET biosynthetic enzymes to confirm ET production. The author did find an increase in fecal ET, and the use of ET in this model improved sleep abnormalities and social avoidance behavior. However, our laboratory’s use of isotope labeled histidine as a precursor did not show any signs of ET biosynthesis. The genomes of Rhodobacillus and Lactobacillus do not seem to contain ET biosynthesis genes. Perhaps the author believes that lactic acid bacteria can accumulate ET from their environment. However, the uptake and utilization of ET by the gut microbiota may still play a crucial role in influencing host uptake of ET. We still need to do more work to study this.
In fact, Escherichia coli can decompose ET to produce thiourea alkanoic acid and trimethylamine, attributed to trimethylamine lyase and lysozyme. Other microorganisms, including Burkholderia. Treponema and Chlorella also possess ergot sulfatase. Further research is needed to evaluate whether other symbiotic bacteria can degrade ET and whether this helps reduce intestinal absorption of ET.
Biosynthetic mechanism of ET
EFSA has allowed the use of ET as a food additive and supplement, even for pregnant women and infants. At the same time, the US Food and Drug Administration has granted ET “Generally Recognized Safety” (GRAS) as a food additive and supplement. This helps ET to be more widely applied to general health and well-being, or to combat a range of diseases.
In addition to chemical synthesis, several groups have demonstrated the biological production of ET in yeast such as Saccharomyces cerevisiae, by cloning two ET biosynthetic genes from mushrooms or a combination of bacterial and fungal genes from Mycobacterium smegmae and fungi. Other research groups have enhanced the copy number of ET biosynthetic genes and increased the availability of histidine precursors through recombination. Other methods have also been used to produce ET using synthetic biology and to expand its production using a range of bacterial and fungal microorganisms, and even to produce isotope labeled ET variants for research.